Dalton’s atomic theory was the experiment done by an English physicist and chemist John Dalton in the year 1808. He wanted to understand the nature of the matter, for that he put forward the theory. During his experiment, he came to know that all matter is made up of particles. Thus here we will be discussing five postulates of Dalton’s atomic theory along with its advantages and disadvantages.
5 Postulates of Dalton’s Atomic Theory
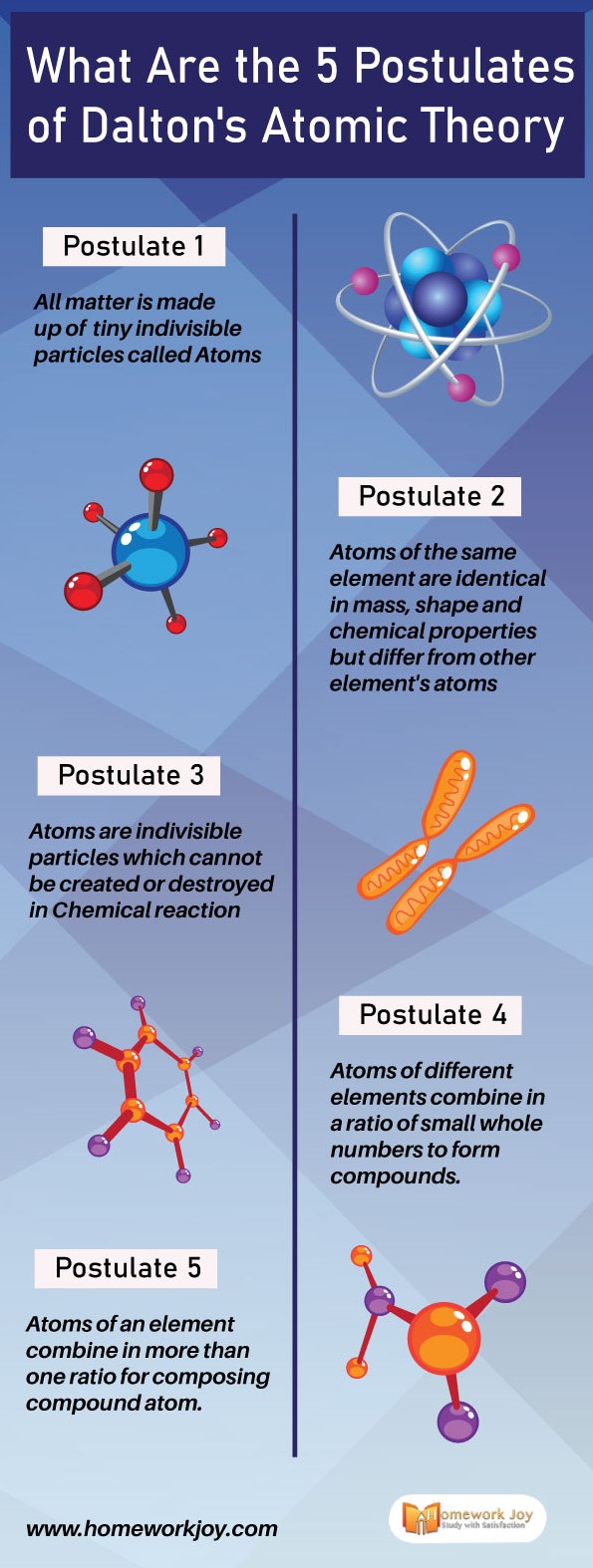
#Postulate1
All matter is made up of tiny indivisible particles called Atoms.
#Postulate 2
Atoms of the same element are identical in mass, shape, and chemical properties. But atoms of other element’s atoms differ in size, shape, and chemical properties.
#Postulate 3
Atoms are indivisible particles which cannot be created or destroyed in Chemical reaction.
#Postulate 4
Atoms of different elements combine in a fixed ratio of small whole numbers to form compounds.
#Postulate 5
Atoms of in a chemical reaction combine or rearrange in more than one ratio for composing compound atom.
Advantages of Dalton’s Atomic Theory
- Dalton’s atomic theory explains the laws of chemical combination that is the law of constant combination and the law of multiple proportions.
- Only due to this theory, the distinction between a fundamental particle that is an atom and the compound particle that is a molecule is put forward.
Drawbacks of Dalton’s Atomic Theory
- The first postulate of Dalton’s atomic theory states that the atoms cannot be further divided. But we have seen that atoms can be divided into neutrons, protons, and electrons. Also, an atom is the smallest particle that participates in a chemical reaction.
- The second postulate states that atoms of the same elements have identical mass, shape, and chemical properties. But there are some atoms that vary in masses and shapes. When the same element has different masses of atoms then it is called isotopes. For example, chlorine has two isotopes with 35 and 37 mass numbers.
- Again in the second postulate, Dalton also mentioned that different atoms have different masses, shapes, and chemical properties. But there are isobars of argon and calcium with mass number 40. It means that isobars are elements which differ from each other but still have the same masses.
- The fourth postulate of his theory states that different elements combine in a fixed whole number ratios to form the compound molecules. However, such a statement is not true in terms of complex organic compounds like sugar (C12H22O11).
So these were five postulates of Dalton’s atomic theory. If you need to know more about other topics in Chemistry, then you can take Chemistry assignment help from our experts.