Chemical reactions are an important part of our lives. Today chemical reactions are contributing to various human activities that we encounter every day, from cooking food to taking a bath. Everything in this natural world is composed of different chemical reactions that we are going to discuss here.
To understand the types of chemical reactions, you must have some basic knowledge of elements and how do they react. So for that check out the link below:
Facts on Periodic Table of Elements
What Are the 5 Postulates of Dalton’s Atomic Theory
Now let’s see different types of chemical reaction in chemistry:
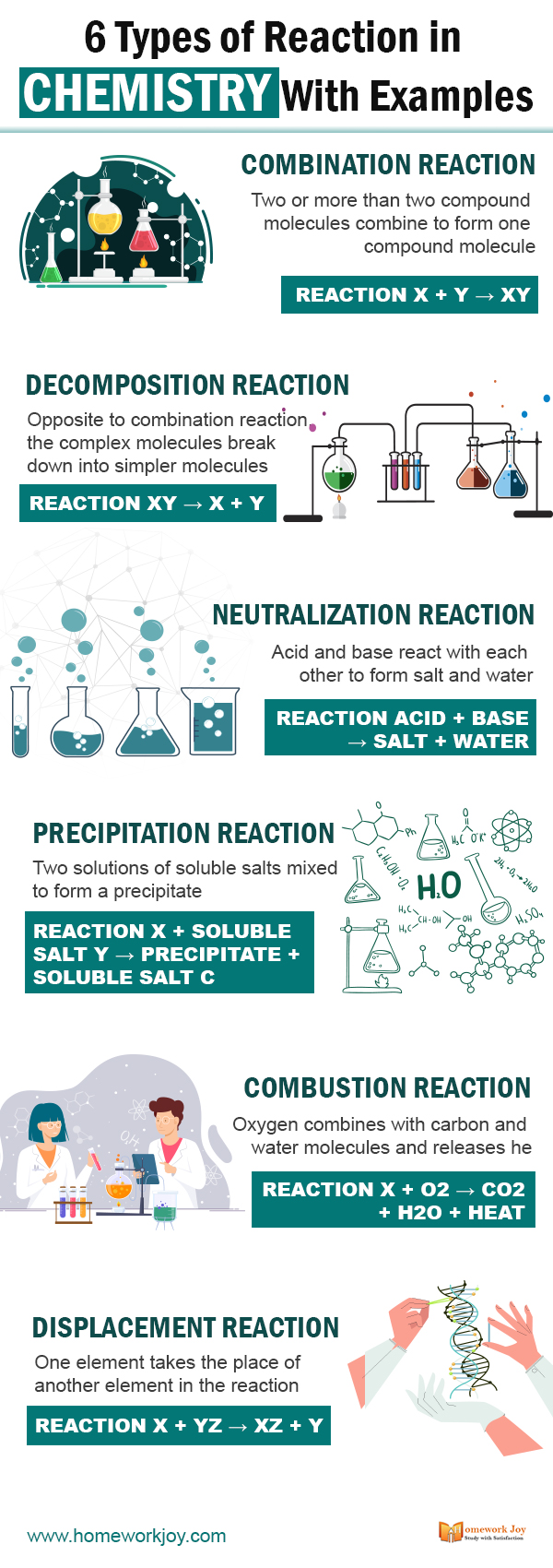
Combination Reaction
Two or more than two compound molecules combine to form one compound molecule.
Reaction
X + Y → XY
There are three types of combination reaction:
- Between two elements
For example, C+ O2 → CO2
- Between two compounds
For example, CaO + H2O → Ca(OH)2
- Between a compound and an element
For example, O + H2O → H2O2
Decomposition Reaction
Opposite to combination reaction, the complex molecules break down into simpler molecules
Reaction
XY → X + Y
Have you ever noticed hydrogen peroxide? It is commonly used as a disinfectant to protect cuts and scratches from bacterial infection. Generally, it is kept in the dark rooms since, in sunlight, it undergoes a chemical reaction. In the daylight, hydrogen peroxide breaks down into water and oxygen. This process is a decomposition reaction.
Neutralization Reaction
Acid and base react with each other to form salt and water; it is known as neutralization reaction.
Reaction
Acid + Base → Salt + water
The term salt here defines the ionic compound that can be either soluble or insoluble. The neutralization reaction is the type of chemical reaction in chemistry that can proceed even if one reactant is not in the aqueous phase. For example,
3 HCl (aq) + Fe(OH)2(s) → 3 H2O (l) + FeCl3 (aq)
Precipitation Reaction
Two solutions of soluble salts mixed to form a precipitate. The precipitation reaction is defined as “Chemical reaction occurring in aqueous solutions where two iconic bonds combine forming up insoluble salts.” From the precipitation reactions, we get salts as precipitates or products of precipitates.
Reaction
X + Soluble Salt Y → Precipitate + Soluble Salt C
Combustion Reaction
Oxygen combines with carbon and water molecules and releases heat. The combustion reactions are high-temperature exothermic types of chemical reactions in chemistry.
Reaction
X + O2 → CO2 + H2O + Heat
For example, combustion of Methane
CH4 (g) + 2 O2 (g) → CO2 (g) + 2 H2O (g)
Displacement Reaction
One element takes the place of another element in the reaction. The displacement reaction occurs only when the metal of electrochemical series mixed with the ions of lower metal in the electrochemical series. The reaction is of two types double displacement reaction and single displacement reaction.
Reaction
X + YZ → XZ + Y
Single Displacement
Cl2 (aq) + 2 NaBr (aq) → 2 NaCl (aq) + Br2 (aq)
Double Displacement
BaCl2 (aq) + Na2So4 (aq) → BaSO4 (s) + 2 NaCl (aq)
So these were some types of chemical reactions in chemistry that we observe in our daily life. If you still have queries or want to know more about it, take instant Chemistry assignment help from our experts.