The laws of physics are the conclusion based on long scientific research and experiments. The experiments are repeated again and again under different circumstances to reach the assumptions that will be accepted worldwide. The world works on certain principles drawn by scientists. The scientific community continuously validates the laws over time.
Over the years, scientists have discovered that nature is very much more complicated than we thought. The laws of physics are considered fundamental because the world works on these laws.
Although there are so many laws in physics, some essential laws of physics are:
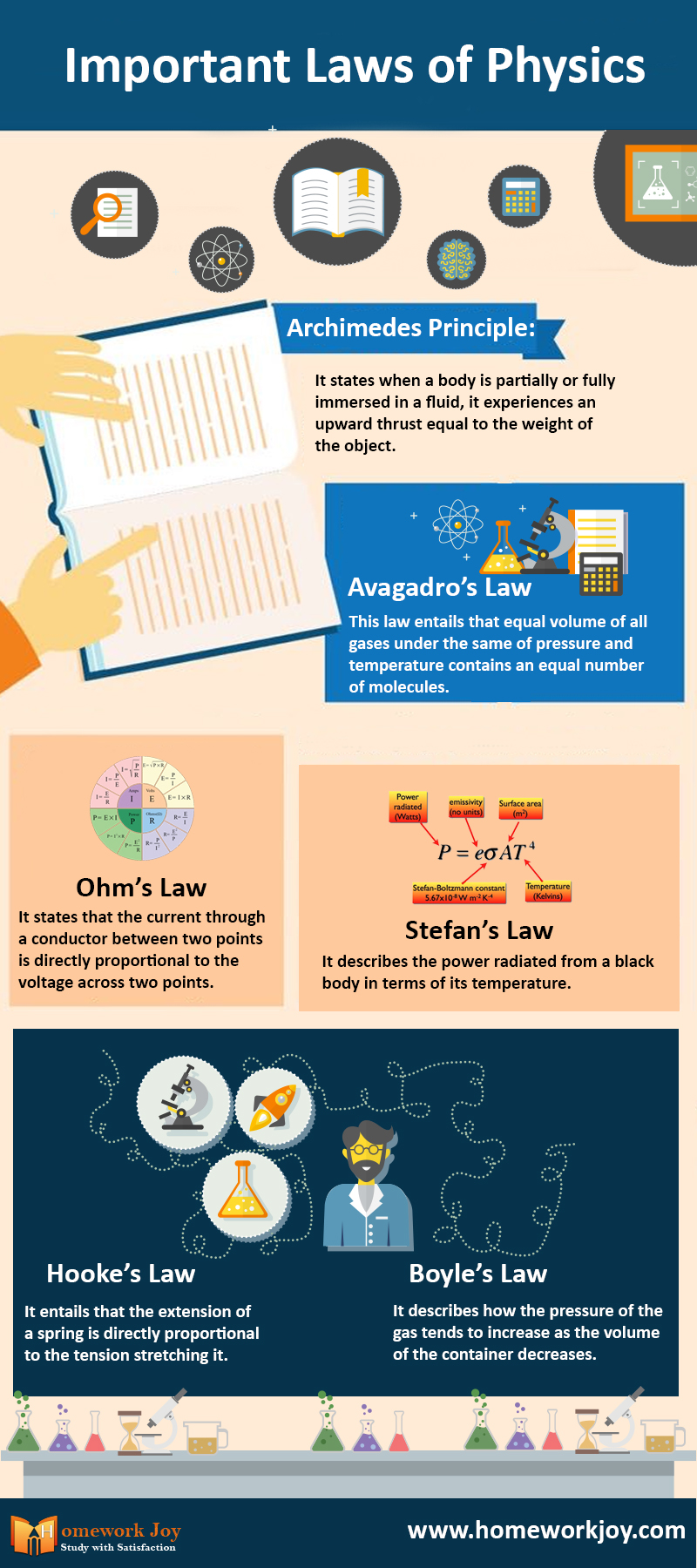
1. Archimedes Principle
It states that when a body is partially or fully immersed in a fluid, it experiences an upward thrust equal to the weight of the object. It means that the apparent loss of weight of the body is equal to the weight of the liquid displaced. Greek mathematician Archimedes discovered it.
2. Avagadro’s Law
This law entails that equal volumes of all gases under the same conditions of pressure and temperature contain an equal number of molecules. Anedeos Avogadro discovered the law. The law is only true for ideal gases.
3. Ohm’s Law
It states that the current through a conductor between two points is directly proportional to the voltage across two points. The constant of proportionality is called resistance and is different from different materials.
4. Stefan’s Law
It describes the power radiated from a black body in terms of its temperature. The constant of proportionality is called Stefan’s constant. Mathematically, P= e Sigma A T4, where e is the emissivity of the object
5. Hooke’s Law
It entails that the extension of a spring is directly proportional to the tension stretching it. Doubling the tension will result in doubling the amount of stretch. Here the constant is the Young modulus of elasticity of the substance.
6. Boyle’s Law
It describes how the pressure of the gas tends to increase as the volume of the container decreases. The law also applies to an ideal gas. Robert Boyle found the law. Real gases follow the law under low pressure.